About periodic table?
Electronegativity and the Periodic Table
Electronegativity can best be thought of as the strength of the pull an atom has to attract a bonding pair of electrons. When two atoms are joined through the action of a pair of shared electrons, this forms a chemical bond known as a covalent bond. Covalent bonds can be equal, or the pull of one atom can be greater than the pull of the other atom, meaning that the electron pair is pulled more towards the atom with the stronger pull and the bond is known as a polar covalent bond. Electronegativity varies across the periodic table in a well understood pattern, meaning that it is possible to use the periodic table of elements to predict the behaviour of different elements in combination (substances made of different elements in combination are known as compounds). If two atoms have very different electronegativity, they will not usually form covalent bonds, instead forming ionic bonds where the valence electron is lost or gained, rather than shared.
What determines electronegativity?
Although electronegativity is only relevant to atoms that are combining with other atoms, i.e. those which are forming a molecule, the factors that control the degree of electronegativity of particular atom are intrinsic to the atom itself. They include three main factors. These are
- How many protons exist in the nucleus of the atom
- How far the valence shell or orbital is from the nucleus of the atom
- The extent to which the electrical pull of the nucleus is screened or shielded by the movement of inner electrons.
All of these factors will have an influence, although the different factors may be influencing the electronegativity in opposite directions.
How is electronegativity measured?
There are several different scales available to measure electronegativity. The most common is the Pauling scale. This takes measurements of dissociation energy (the amount of energy change when a bond is released) for a particular element and provides a measure of the difference in electronegativity between different elements.
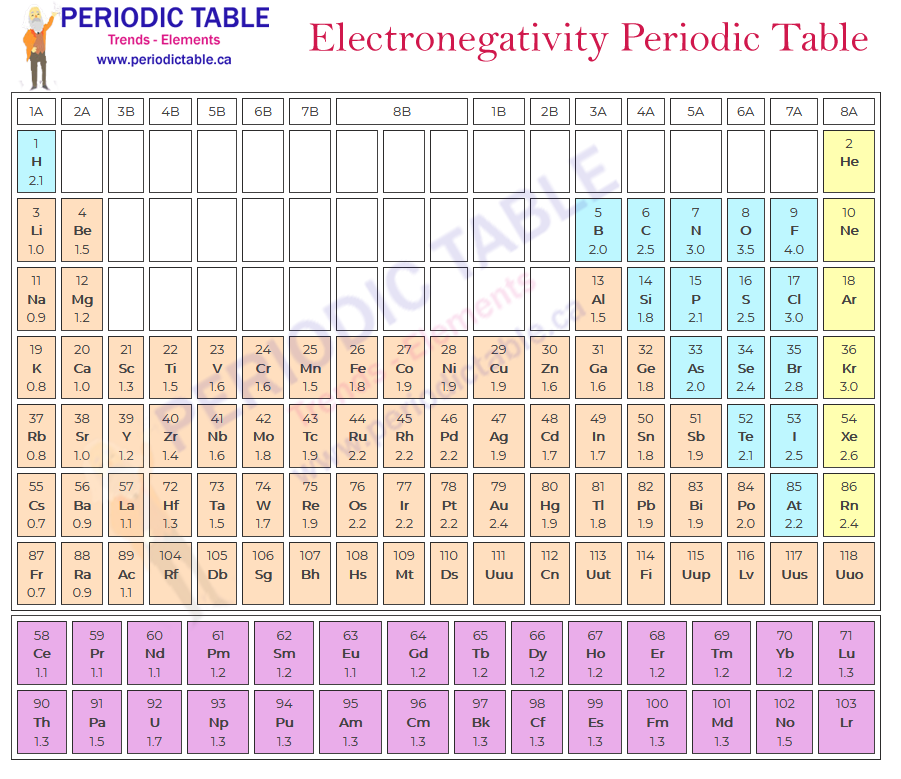
Because the Pauling scale provides a measure of the difference in electronegativity between different elements, it has been necessary to select a single fixed point to allow elements to be assigned a single value, independent of their relationship to each other. Hydrogen has been designated the reference point and has an electronegativity score of 2.20 on the Pauling scale. Overall, the most electronegative element is fluorine, which has a value of 4.00 on the Pauling scale. This compares with a score of 0.70 given to francium, which is the least electronegative.
How does electronegativity vary across periods in the periodic table?
Rows in the periodic table are known as periods. When looking at the table, electronegativity increases as you move from left to right across a single row. This is because the number of protons increases as you move across the periods. When electron pairs are shared between elements, they will be more attracted to the element with the greater number of protons, meaning that this atom will be the more electronegative of the pair, if all else is equal.
How does electronegativity vary within a group in the periodic table?
When looking at the periodic table, the columns are known as groups. Electronegativity decreases as you move down the group. This is because all of the elements in the same group have the same number of valence electrons. This, if an element is in group 1, it only has one valence electron and, therefore when a covalent bond is formed, there is the same number of shared electron pairs in each of the elements in the group.
In contrast, the distance between the outer valence electron shell and the nucleus increases as you move down the group. This not only reduces the pull from the protons, but it also increases the number of electrons that shield the nucleus. This reduces the electronegativity of the element.