Information about periodic table?
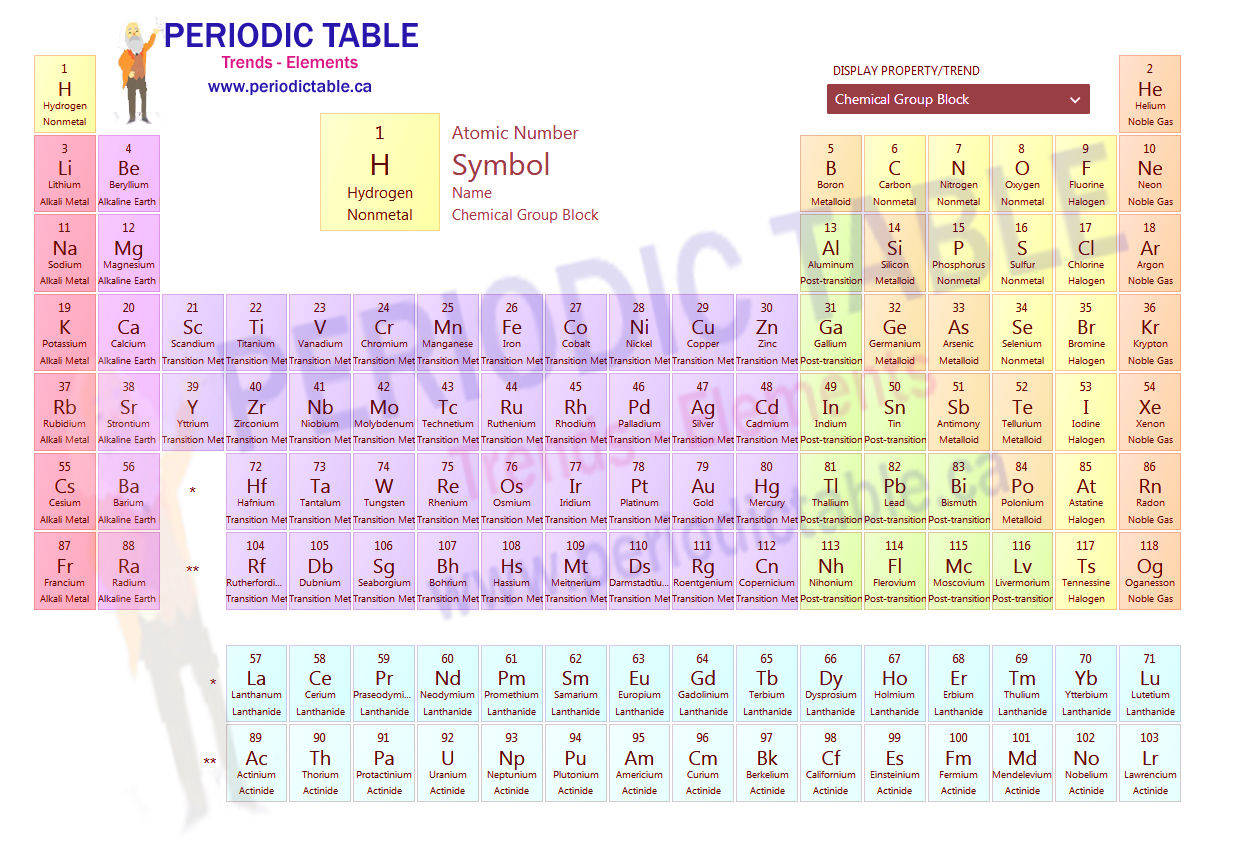
Periodic Table of Elements
The periodic table of elements (often referred to as just ‘the periodic table’) is the way in which scientists categorise and organise the different elements used in chemistry. It carries a great deal of information which allows scientists to predict the behaviour of different elements in terms of their ability to form compounds with other elements and the way in which they are going to react.
How the periodic table of elements was created
The periodic table of elements was created in the 1800’s when researchers noted that there were patterns in the reactivity and characteristics of elements when you organised them by atomic mass. The main researcher credited with the creation of the table was Mendeleev, but several other researchers were working on similar structures at the same time. The thing that made Mendeleev’s structure stand out was the fact that he left space for elements that had not been discovered yet but whose existence were suggested by the structure of the rest of the table.
The physics underlying the structure of the periodic table of elements
Although the periodic table of elements was initially created based on atomic mass, this is based on a combination of the weights of protons and neutron in the nucleus of the individual elements. Several elements were placed in a position on the table that was different to that dictated by their atomic mass (because their characteristics fit better in the alternative position). Once the atomic structure was fully understood, it became clear that the position of any element in the periodic table of elements was based on the number of protons in the nucleus and, as a result, by the number of electrons found in the outer shell of electrons. This is known as the number of valence electrons. Different columns (known as groups) in the periodic table of elements have similar characteristics, but they also have the same number of valence electrons.
The number of valence electrons an element has is the reason behind the shared characteristics. This is because the number of valence electrons dictates how reactive the element is and the way it behaves.
The structure of the periodic table of elements
The periodic table of elements is divided into groups and periods. The horizontal rows in the periodic table of elements are known as periods. Columns in the table are referred to as groups. Elements within a group are very similar. They have the same number of valence electrons, meaning that they have very similar levels and patterns of reactivity.
Trends within periodic table of elements
The periodic table of elements shows clear patterns and trends across the elements listed within it. These patterns and trends are what gives the table its predictive quality, which is where the value of the table comes from.
Elements further down the column have a larger nucleus than those towards the top of the column, and their valence shells are further away from the nucleus, meaning that it is easier for these larger atoms to lose electrons than the smaller atoms. In contrast, it is harder for the larger atoms to gain electrons. This means that metallic elements (which need to lose electrons to react) become more reactive as you go down the group whilst those which need to gain an electron (non-metallic elements) become less reactive.
Elements added to the periodic table of elements
Since the initial version of the periodic table of elements in the 1800’s, many elements have been added to it. Some of these were the elements predicted by Mendeleev when he first formulated the table. The final group in the table is the noble gases, none of which had been discovered when the table was created. As further research has been concluded, new synthetic elements have also been added to the table.